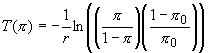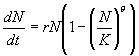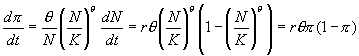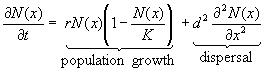The Origins of Agriculture as a Natural Experiment in Cultural
Evolution
Peter J. Richerson, Robert Boyd, and Robert L. Bettinger
Do Not Cite In Any Context Without Permission Of Authors
Peter J. Richerson, Department of Environmental Science and Policy, University
of California—Davis, Davis, CA 95616, pjricherson@ucdavis.edu
Robert Boyd, Anthropology Department, University of California—Los Angeles,
Los Angeles, CA 90024, rboyd@anthro.ucla.edu
Robert L. Bettinger, Anthropology Department, University of California—Davis,
Davis, CA 95616, rlbettinger@ucdavis.edu
Abstract: Several independent trajectories of subsistence
intensification, often leading to agriculture, began during the Holocene.
No plant rich intensifications are known from the Pleistocene, even from
the late Pleistocene when human populations were otherwise quite sophisticated.
Recent data from ice core climate proxies show that last glacial climates
were extremely hostile to agriculture—dry, low in atmospheric CO2,
and extremely variable on quite short time scales. We argue that agriculture
was impossible under last-glacial conditions. The quite abrupt final amelioration
of the climate was followed immediately by the beginnings of plant intensive
resource use strategies in some areas, although the turn to plants was
much later elsewhere. Almost all trajectories of subsistence intensification
in the Holocene are progressive and eventually agriculture became the dominant
strategy in all but marginal environments. In the Holocene, agriculture
is, in the long run, compulsory. We use a mathematical analysis to show
that the rate limiting process for intensification trajectories must almost
always be the rate of innovation of subsistence technology or subsistence
related social organization. At the observed rates of innovation, population
growth will always be rapid enough to sustain a high level of population
pressure. Several processes appear to retard rates of cultural evolution
below the maxima we observe in the most favorable cases.
Resumen: Varias trayectorias independenties de la intensificación
del sustento , muchas de las cuales conducieron a la agricultura, empezaron
durante el Holoceno. No conocemos ninguna intensificació que usara
recursos vegetales durante el Pleistoceno, inclusive el Pleistoceno último,
cuando las poblaciones humanas fueron muy sofisticadas en otros ámbitos.
Datos recientes de cilindros de hielo sacados de Groenlandia muestran que
la última glaciación fué extremadamente hostil a la
agricultura, ya que fué—seca, baja en CO2, y extremadamente
variable en el corto plazo. Proponemos que la agricultura fué imposible
en estas condiciones de la última glaciación. La súbita
mejora del clima al final de la glaciación fué seguida inmediatamente
por la iniciación de usos intensivos de los recursos vegetales en
algunos lugares, aunque mucho mas tarde en otras partes. Casi todo las
trajectores de intensificación en el Holoceno eran occurrieron sin
retroceso. Finalmente, la agricultura se convirtió en el modo principal
de sustento en todas partes, excepto por zonas muy frías o muy secas.
En el Holoceno, la agricultura se vuelve, a final de cuentas, obligatoria.
Usamos un análisis matemático para mostrar que los procesos
limitante de la taza de intensificación debe ser casi siempre la
taza de la inovación tecnológica en las estrategias de sustento,
o la taza de inovación en las formas de organazación social
en relación al sustento. Con las tazas de inovación que se
observan, el crecimiento de la población siempre es suficientemente
rapido como para crear alto nivel de presión poblacional. Al parecer
, varios processos normalmente retardan la velocidad de la evolución
cultural abajo de las tazas máximas que observamos en el modelo.
While observing the barbarous inhabitants of Tierra del Fuego, it struck
me that the possession of some property, a fixed abode, and the union of
many families under a chief, were the indispensable requisites for civilization.
Such habits almost necessitate the cultivation of the ground; and the first
steps would probably result from some such accident as the seeds of a fruit
tree falling on a heap of refuse, and producing some unusually fine variety.
The problem, however, of the first advance of savages toward civilization
is at present much too difficult to be solved.
Charles Darwin Descent of Man 1874
Evolutionary thinkers have long been fascinated by the origin of
agriculture. While Darwin declined to speculate on agricultural origins,
Twentieth Century scholars were bolder. The Soviet agronomist Nikolai Vavilov,
American geographer Carl O. Sauer, and British archaeologist V. Gordon
Childe wrote influential books and papers on the origins of agriculture
in the 1920s and 30s (see MacNeish 1991: 4-19, for a discussion of the
intellectual history of the origins of agriculture question). These explorations
were necessarily speculative and vague, but they stimulated interest. Vavilov
and Sauer argued that agriculture originated in locations where the wild
ancestors of later crop species attracted the attention of hunters and
gatherers, leading eventually to domestication. Childe argued that climate
change accompanying the end of the Pleistocene was responsible for the
initial steps that led to domesticated crops.
Immediately after World War II, the American archaeologist Robert Braidwood
(Braidwood, et al. 1983) pioneered the systematic study of agricultural
origins. From the known antiquity of village sites in the Near East and
from the presence of wild ancestor species of many crops and animal domesticates
in the same region, Braidwood inferred that this area was likely a locus
of early domestication. He settled on a region in the foothills of the
southern Zagros Mountains in Iraqi Kurdistan as a likely place for domestication
to have begun. He then embarked on an ambitious program of excavation using
a multi-disciplinary team of archaeologists, botanists, zoologists, and
earth scientists to extract the maximum useful information from the excavations.
The availability of 14C dating gave his team a powerful tool
for determining the ages of the sites.
The Braidwood team’s main effort focussed on the Jarmo site, but excavation
at a site nearby uncovered an earlier settlement that had been occupied
seasonally by hunter-gatherers who were collecting wild seeds, probably
the ancestors of wheat and barley, and hunting the wild ancestors of goats
and sheep about 11,000 years ago. (Ages are given here as calendar dates
before present (B.P.), where present is taken to be 1950, estimated from
14C dates according to Stuiver, et al.’s (1998) calibration
curves.) Near Eastern sites older than about 15,000 B.P. excavated by Braidwood
(Braidwood and Howe 1960) and others were occupied by hunter-gatherers
who put much more emphasis on hunting and much less on collecting and processing
seeds (Henry 1989; Goring-Morris and Belfer-Cohen 1998). At Jarmo itself,
the team excavated an early farming village dating to about 9,000 B. P.
Using much the same seed processing technology as their immediate gathering
predecessors, the Jarmo people no longer moved their residences with the
seasons. Analyses of plant and animal remains suggested that the process
of domestication was underway. This early agricultural village is at the
base of an archaeological record of larger and increasingly sophisticated
agrarian settlements that characterize the Near Eastern archaeological
record leading to the first state level societies in Mesopotamia about
5,500 BP.
Since the pioneering Jarmo excavations, a few dozen multi-disciplinary
archaeological teams, have studied likely sites of agricultural transitions
in the Near East, North and South America, the Far East, and Sub-Saharan
Africa. At least six regions (Table 1) were autonomous centers of plant
and/or animal domestication, and incomplete, controversial evidence is
taken by some scholars to imply at least two more. Ehret (1998) argues
on grounds of “linguistic archaeology” that Saharan and Sub-Saharan Africa
had three independent centers of domestication rather than the one more
commonly accepted. Historical linguists have reconstructed three sets of
agricultural vocabulary uncontaminated by loan-words, implying independent,
rather early, episodes of domestication. These investigations have discovered
no region in which agriculture developed earlier or faster than in the
Middle East, though a North Chinese center of domestication of millet may
prove almost as early (see discussion below). Indeed other centers seem
to have developed later, or more slowly, or with a different sequence of
stages, or all three. The spread of agriculture from centers of origin
to more remote areas is well documented for Europe and North America. Ethnography
also gives us cases where hunters and gathers persisted to recent times
in areas seemingly highly suitable for agriculture, most notably much of
western North America and Australia. Attempts to account for this rather
complex pattern are a major focus of archaeology.
Origin of agriculture is a macroevolutionary “experiment”
“Macroevolution” is the evolutionary biologist’s term for the large scale
and long-term pattern of evolution. The contrasting term, “microevolution,”
refers to generation-to-generation changes. Because much important evolutionary
change takes place at macroevolutionary time scales, it is important for
evolutionary theory to explain such changes. Unfortunately, while small-scale
microevolutionary changes are accessible to direct observation and critical
experiment, processes that act on long time scales are not. Thus a major
project for students of both organic and cultural evolution is to link
theory derived from microevolutionary studies to the realm of macroevolutionary
events.
Because evolving systems are normally complex, and data that speak to the
past are so sparse, testing evolutionary hypotheses with macroevolutionary
data is difficult. The best cases to work with are those where large, simple,
changes are imposed upon the system. Large changes ensure that the system’s
evolutionary response to the change will not be lost in the welter of unknown
causes of change that will ordinarily make past events exceedingly difficult
to understand. Simple changes make it easier to follow the inevitably complex
chains of cause and effect in history. The classical laboratory experiment,
similarly, depends upon making large, simple (controlled) manipulations
to disentangle complex causal processes. Subtlety comes only when basic
processes are very well understood.
The several independent origins of agriculture provide just such a case.
They all occurred relatively recently and began rather abruptly. Agricultural
developments occurred at least 90 millennia after the last major biological
change evidenced in the fossil record, the evolution of anatomically modern
humans. They occurred about 30 millennia after human cultural artifacts
reach a level of stylistic and technical sophistication that convincingly
demonstrates that people were cognitively modern as well. Our argument
here is that a near step-function change in the earth’s environment from
Pleistocene to Holocene climatic conditions about 11,600 years ago transformed
the world from a place where agriculture was impossible anywhere to a place
where it was possible on a large fraction of the earth’s surface. The various
trajectories of agricultural origin and spread in different parts of the
world thus result from a single, strong, “manipulation.” The replication
of origins and spreads under different local conditions give us variation
in additional factors. In particular, the great variation in rates of progress
toward agriculture and in the rates of increase of sophistication of agriculture
after its initial development give us insight into the processes which
govern the rate of cultural evolution.
Cultural evolution is best studied using Darwinian methods
The division of labor in the social sciences between economists, sociologists,
and ethnographers on the one hand, and historians and archaeologists on
the other, is very similar to the division of labor between paleontologists
and students of microevolution. However, formal evolutionary theory is
less well developed in the social sciences than in biology. The conceit
of this paper is that thinking about social science problems, like the
origins of agriculture, in the way that biologists think about analogous
problems is a useful approach. Boyd and Richerson (1985) have developed
this project mainly through investigating formal theoretical models of
cultural evolution. Bettinger (1991; Bettinger and Eerkens 1999) has addressed
the application of such theory to archaeological problems. The models are
built on the idea that the transmission of culture by imitation and teaching
is analogous to the transmission of genes. Cultural traditions, like genetically
transmitted traits, are a population level phenomenon. The ideas we inherit
culturally are a sample of those that characterize the larger population
we live among. To a large extent, we are the prisoners of our cultural
history, just as we are prisoners of the genes we inherit from our parents.
At the same time, individuals (and more problematically groups of individuals)
are the locus of the processes that are currently transforming cultural
traditions into something new. If living individuals are the prisoners
of the past they are also the architects of the future. Darwinian theory
is, at its most formal, a calculus for analyzing change in an immortal
population of mortal individuals when important state variables of the
system are the result of the transmission of information from individual
to individual.
The biological theory already makes room for a rather long list of structural
alternatives in the nature of inheritance systems and for a long list of
“forces” that generate evolutionary dynamics. To apply the calculus to
culture requires expanding both lists to take account of such things as
the possibility of having more than two “parents” in cultural transmission.
More fundamentally, we have to recognize that people actively and purposefully
“engineer” the cultural traditions they inherit in a way that has no analogy
in organic evolution. This expansion leads to many interesting differences
between genetic and cultural evolution. Of the greatest significance is
that cultural evolution is more rapid than genetic evolution. When the
results of individual learning and choices among alternative cultural variants
are transmitted to others by teaching or imitation, the potential for higher
rates of change is obvious. To the extent that invention and choice are
guided by adaptive rules, these processes will act to favor the same variants
as selection, and rates of acquisition of new adaptations will be higher
for a cultural than for a purely genetic system. This makes cultural traditions
superior to organic adaptations when environments are rapidly changing
or highly variable in space. We believe that the cultural transmission
system arose in humans as an adaptation to the highly variable climate
regime of the last few million years (Richerson and Boyd 2000).
Macroevolution is Interesting and Important
Macroevolution is of particular interest when some evolutionary processes
have such long time scales that they cannot not satisfactorily resolved
by microevolutionary investigation. Such enigmatic, long time scale patterns
are evident in both organic and cultural evolution. For example, in organic
evolution, theory suggests and observations confirm, natural selection
can easily lead to appreciable genetic change from one generation to the
next (Endler 1986). In the case of artificial selection, huge differences
can arise in a relatively few generations, as in domesticated breeds like
dogs. The rate at which selection can fill up a new lake with a flock of
new species is, under favorable conditions, measured in thousands of years
(Johnson, et al. 1998). Yet, we know from the fossil record that long-term
change is typically much, much slower. Even rather rapid changes, such
as the expansion of brain size in Homo during the last 2 million
years, would be undetectable by even the most sophisticated microevolutionary
observations. In the case of cultural evolution, we live in a period in
which change is easily appreciable from generation to generation. Invention
is piled upon invention, leading to rapid technological change. Evolution
of social institutions is also very fast. But the modern era is extraordinary.
The Near Eastern trajectory of agricultural innovation was also comparatively
rapid, but the whole sequence of increasing dependence upon plants and
then upon domesticated plants and animals leading to nearly complete dependence
on domesticates occupied roughly 4,000 years. At the average rate of change,
it is unlikely that individuals living around Jarmo could possibly have
been aware of the extraordinary evolutionary trajectory that they were
on. On the other hand, perhaps there were periods of intense innovation
alternating with periods of stagnation, such that, like ourselves, people
were acutely aware of change during the periods of innovation. Or, perhaps,
change is always rapid, but largely random in direction relative to the
long-term trend. The Jarmo people, for example, might have always been
aware of rapid change, but mostly alternating back and forth between more
and less dependence on crops as (say) the primitive, unstable, social institutions
necessary for settled life struggled to emerge, only to collapse in a few
generations. We know from later examples, such as the collapse of the Mayan
city-states, that rapid growth followed by rapid decline is not unusual
(Tainter 1988).
Thus, what we discover about evolution from investigating short-term processes
often does not fit comfortably with what we know of the long-term trajectory
of evolution (Boyd and Richerson 1992). The pioneering attempt to reconcile
paleontology with the mainly microevolutionary theory of the mid-century
Neo-Darwinian Synthesis was George Gaylord Simpson’s (1944) Tempo and
Mode in Evolution. The difficulty of this project is illustrated by
the storm of controversy that arose in evolutionary biology following Eldredge
and Gould’s (1972) proposal that most evolution occurred in unusual punctuational
events accompanying speciation, followed by selection among the resulting
species. The claim was that the species selection process that dominates
long-term evolution is decoupled from minor microevolutionary changes that
evolutionary biologists can study. The debate became heated. Acerbic critics
came to refer to the punctuation hypothesis as “the theory of evolution
by jerks.” Test of the species selection hypothesis have not been kind
to it (Carroll 1997; Levinton 1988), but the controversy has had the effect
of focussing attention on the problem of explaining why evolution seems
fast to “neontologists” but slow to paleontologists.
In the social sciences the debate is equally acrimonious, and the theoretical
gap even worse. At one extreme, rational choice theorists use sophisticated
but completely ahistorical models explain human behavior. At the other,
historians and cultural anthropologists describe historical change in compulsively
detailed but atheoretical terms. Darwinian models of cultural evolution
should help to address such questions. They incorporate the rational choice
theorist’s decision-making effects and the historian’s transmission of
traditions in the same models. The question becomes: What regulates the
tempo and mode of cultural evolution?
Macroevolutionary hypotheses come in two flavors
There are two kinds of hypotheses we can invoke to explain the tempo and
mode of Darwinian evolution (now counting cultural evolution as a type
of Darwinian evolution). First, rates of long-term change may be regulated
by factors external to the evolutionary process itself. We know
that the earth itself is an evolving system, so the environment in which
evolution is occurring is always changing. Continents move, climates change,
and organisms evolve in response. In a classic paper Valentine and Moores
(1970) argued that the long-term pattern of evolution is at least partly
regulated by the geographical and geochemical changes of the earth caused
by seafloor spreading. Seafloor spreading causes slow trends in geochemistry
and topography, and these slow changes often trigger abrupt switches from
one stable regime of the ocean-atmosphere system to another. Natural selection
is a rapid process on the geological time scale, so most of what paleontologists
see is organic evolution tightly tracking environmental change caused by
geological processes. The tempo of organic evolution is regulated
by environmental change and the main mode of change is the ordinary
adaptive evolution by natural selection that neontologists study. Since
cultural evolution is an even more rapid process, the argument should be
even stronger in that case.
Second, internal processes limit the rate and direction of evolution.
Even though selection is a very potent force on the geological time scale,
it is by no means instantaneous. Some lag must exist between current environments
and current organisms unless environmental change is vanishingly slow.
For example, the poorly understood process of speciation limits the rate
of evolution of new species bearing major new adaptations. Speciation is
slow, many evolutionists believe, because geographical accidents must first
isolate new proto-species. Otherwise, interbreeding with the mass of populations
still adapted to the old niche swamps the effect of selection acting to
reshape adaptations in a population that might otherwise enter a new niche.
The number of species extant at any one time will be fewer than can potentially
exist if environmental change is fast enough to prevent evolution from
reaching the equilibrium number. Walker and Valentine (1984) estimated
that on average the number of shelly marine invertebrate species is about
70% of the number that would occur if environmental change stopped long
enough for equilibrium to be reached. The emergence of agriculture, and
the subsequent evolution of complex societies over the last 10,000 years,
are patterns that may well reflect mainly the impact of internal limitations
to the rate and direction of cultural evolution, analogous to the limitations
that speciation seems to impose on the rate of organic evolution. If so,
understanding what these rate limiting process or processes are is fundamental
to understanding how human history works.
Climate Change Started the Experiment
How “clean” is the agricultural origins natural experiment? One possibility
is that climate and other environmental factors that influence agricultural
origins change in complex ways. Indeed, many of the external hypotheses
invoked to explain agricultural origins appeal to detailed, local changes
that imply that external influences were very complex. Childe (1951) argued
that terminal Pleistocene desiccation crowded human populations around
Near Eastern oases where more intensive land uses had to be developed to
support the population, leading to agriculture. Wright (1977), finding
that climate in the Near East became warmer and rainier around the time
of plant domestication, argued that climate change brought together the
suite of plants suitable for domestication in the Fertile Crescent area
where the pioneering domestications took place. Since climate is always
changing, at least a little bit, other climate changes could act as a trigger
in the pioneering domestication sequence in other areas as well. Or other
factors may be more important elsewhere even if climate was the key in
the Near East. If the role of climate change is sufficiently complex, external
and internal factors will be entangled in an even more complex picture.
Climate change will have produced a much better natural experiment if it
changed in one dramatic global episode and then stopped changing. If so,
a large number of replicate human populations were set on a new evolutionary
trajectory by the external step change, but all of the subsequent changes
were due to internal processes or at least to other external processes.
The many replicate populations faced different impediments to the evolution
of agriculture and thus give us clues about what the internal rate limiting
processes are. As it turns out, climate change does appear to provide the
requisite conditions for such a clean experiment.
Agriculture was impossible in the Pleistocene
The Pleistocene geological epoch is characterized by dramatic glacial advances
and retreats. Using a variety of indirect measures of past temperature,
rainfall, ice volume, and the like, mostly from cores of ocean sediments,
lake sediments, and ice caps, paleoclimatologists have constructed a stunning
picture of climate deterioration over the last 14 million years (Lamb 1977;
and Crowley and North 1991; Partridge, et al. 1995; Bradley 1999). The
Earth’s mean temperature has dropped several degrees and the amplitudes
of fluctuations in rainfall and temperature have increased. For reasons
that are as yet ill understood, glaciers wax and wane in concert with changes
in ocean circulation, carbon dioxide, methane and dust content of the atmosphere,
and changes in average precipitation and the distribution of precipitation.
The resulting pattern of fluctuation in climate is very complex. As the
deterioration has proceeded, different cyclical patterns of glacial advance
and retreat involving all these variables have dominated the pattern. A
21,700 year cycle dominated the early part of the period, a 41,000 year
cycle between about 3 and 1 million years ago, and a 95,800 year cycle
during the last million years.
This cyclic variation is very slow with respect to rates of cultural evolution.
As the data from Jarmo indicate, major changes in subsistence take a few
thousand years to complete, but not 20,000 years or more. The glacial cycle
variation will not generate clean experiments because genetic and cultural
evolutionary process could both be important on such long time scales.
However, the variability that occurred on the time scales of the major
glacial advances and retreats is also correlated with great variance at
much shorter time scales. For the last 120,000 years, very high-resolution
data are available from ice cores taken from the deep ice sheets of Greenland
and Antarctica. Resolution of events lasting little more than a decade
is possible in ice 90,000 years old, improving to monthly after 3,000 years
ago. During the last glacial, the ice core data show that the climate was
highly variable on time scales of centuries to millennia (GRIP 1993; Clark
et al. 1999; Ditlevsen, et al. 1996).
Figure 1 shows the data from the GRIP Greenland core. The 18O
curve is a proxy for temperature; less negative values are warmer. Ca2+
is a measure of the amount of dust in the core, which in turn reflects
the prevalence of dust-producing arid climates. The figure also shows histograms
illustrating the obvious. The last glacial period was arid and extremely
variable compared to the Holocene. Sharp excursions lasting a millennium
or so occur in estimated temperatures, atmospheric dust, and greenhouse
gases. The intense variability of the last glacial carries right down to
the limits of the nearly 10 year resolution of the ice core data. Figure
2 shows Ditlevsen et al.’s (1996) analysis of a Greenland ice core. Not
only was the last glacial much more variable on time scales of a century
or more (150 yr low pass filter) but also on much shorter time scales (150
yr high pass filter). Even though diffusion within the ice core progressively
erases high frequency variation in the core, the shift from full glacial
conditions about 18,000 years ago to the Holocene interglacial is accompanied
by a dramatic reduction in high frequency variation. The Holocene (the
last relatively warm, ice free 11,600 years) has been a period of very
stable climate, at least by the standards of the last glacial.
Holocene weather extremes have significantly affected agricultural production
(Lamb 1977). It is hard to imagine the impact of the qualitatively greater
variation that characterized of most if not all of the Pleistocene. Devastating
floods, droughts, windstorms, and the like, which we experience once a
century, might have occurred once a decade. Tropical organisms did not
escape the impact of this climate variation; temperature and especially
rainfall were highly variable at low latitudes (Broecker 1996). Plant and
animal populations responded to climatic change by dramatically shifting
their ranges, but climate change was significant on the time scales shorter
than those necessary for range shifts to occur. As a result, natural communities
must have always been in the process chaotic reorganization as the climate
varied more rapidly that they could reach equilibrium. The pollen record
from the Mediterranean illustrates how much more dynamic plant communities
were during the last glacial (Allen, et al. 1999).
In addition to fluctuations in temperature and rainfall, the CO2
content of the atmosphere was about 190 ppm during the last glacial, rising
to about 250 ppm at the beginning of the Holocene (Figure 3). Photosynthesis
on earth is CO2 limited over this range of variation (Sage 1995).
During the last glacial period, seed yields may have been something like
2/3s of Holocene yields. Nothing appears to be known about the evolutionary
responses of plants to lower CO2 concentrations. If low CO2
caused plants to allocate more photosynthate to vegetative rather than
reproductive tissues like seeds or storage organs like tubers, then the
impact on low CO2 on the attractiveness of plant foods might
be underestimated by the data reviewed by Sage.
During the last glacial, at least, people lived under environmental conditions
that almost certainly made heavy dependence on food plants unattractive.
On present evidence we cannot determine whether aridity, low CO2,
or climate variability is the main culprit in preventing the evolution
of agriculture. Low CO2 and climate variation would handicap
the evolution of dependence on plant foods everywhere. Plant food rich
diets take considerable time to develop. Processing technology has to be
invented and made efficient. Plant foods are generally low in protein and
often high in toxins. Some time is required to work out a balanced diet
of plant foods. The direct archaeological evidence suggests that people
began to use extensively the technologies that underpinned agriculture
only after about 15,000 B.P. (Bettinger in press). As CO2 levels
rose, climate variability decreased, and rainfall increased, human populations
in several parts of the world began to turn to the exploitation of locally
abundant plant resources, but only during the so-called Bølling-Allerød
period of near interglacial warmth and stability. One last siege of glacial
climate, the Younger Dryas from 12,900 yrs B.P. until 11,600 yrs B.P.,
probably reversed these adaptive trends (e.g., Goring-Morris and Belfer-Cohen
1998). The Younger Dryas climate was appreciably more variable than the
preceding Allerød-Bølling and the succeeding Holocene (Grafenstein,
et al. 1999; Mayewski, et al. 1993). The ten abrupt, short, warm-cold cycles
that punctuate the Younger Dryas ice record were probably felt as dramatic
climate shifts all around the world. After 11,600, the Holocene period
of relatively very warm, wet, stable, CO2 rich environments
began. Subsistence intensification and eventually agriculture followed.
Thus, while not a perfectly instantaneous change, the shift from glacial
to Holocene climates was a very large change, and took place much more
rapidly than cultural evolution could track.
Might we not expect agriculture to have emerged in the last interglacial
130,000 years ago or even during one of the even older interglacials? No
archaeological evidence has come to light suggesting the presence of complex
technologies that might be expected to accompany forays into intensive
plant collecting or agriculture at this time. The human populations of
the last interglacial were still archaic in anatomy and behavior, though
the first anatomically (but not yet culturally) modern humans occur in
the archaeological record just after 130,000 years ago (Klein 1999: Ch.7).
Very likely, then, the humans of the last interglacial were neither cognitively
nor culturally preadapted for the evolution of agricultural subsistence.
We should point out, however, that an external explanation might also explain
the lack of agriculture during previous interglacials. Ice core data from
the thick Antarctic ice cap at Vostok show that each of the last four interglacials
over the last 420,000 years was characterized by a short, sharp peak of
warmth, rather than the 11,500 year long stable plateau of the Holocene
(Petit, et al. 1999). Further, the GRIP ice core suggests the last interglacial
(130-80,000 B.P.) was more variable than the Holocene although its lack
of agreement with a nearby replicate core for this time period makes this
interpretation tenuous. On the other hand, the atmospheric concentration
of CO2 was higher than during the Holocene in the three previous
interglacials, and was stable at high levels for about 20,000 years following
the warm peak during the last interglacial. The highly continental Vostok
site unfortunately does not record the same high frequency variation in
the climate as most other proxy climate records, even those in the Southern
Hemisphere (Steig, et al. 1998). Some Northern Hemisphere marine and terrestrial
records suggest that the Last Interglacial was highly variable while other
data from suggest a Holocene length period of stable climates ca. 117,000-127,000
B.P. (Frogley, et al. 1999). In sum, better data on the high frequency
part of the Pleistocene beyond the reach of the Greenland ice cores is
needed to test hypotheses about human macroevolutionary events antedating
the latest Pleistocene. That aside, the comparatively primitive nature
of human adaptation during the last interglacial seems sufficient by itself
to account for the observed lack of agricultural experimentation then.
In the Holocene, agriculture is compulsory in the long run
Once a more intensive subsistence system is possible, it will, over the
long run, replace the less intensive subsistence system that preceded it.
The reason is simple: all else equal, any group that can use a tract of
land more efficiently will be able to evict residents that use it less
efficiently. More intensive uses support higher population densities, or
wealthier societies per capita, or both. An agricultural frontier will
tend to expand at the expense of hunters and gatherers as rising population
densities on the farming side of the frontier motivate pioneers to invest
in acquiring land from less efficient users. Whether the competition for
land is economic, military, or for social prestige, the hunter-gatherer
will be offered an attractive purchase price, dismal choices between flight,
submission, or military defense at long odds against a more numerous foe,
or an attractive idea about how to become richer through farming. Subsistence
improvement generates both literal and metaphorical arms races. The archaeology
supports this argument (Bettinger in press). Societies in all regions of
the world undergo a very similar pattern of subsistence intensification
in the Holocene, albeit at very different rates. Since ever more intensive
subsistence systems have continued to evolve right up to the present, we
do not have to worry about any very significant relaxation in the selection
pressure for more efficient subsistence during the Holocene. A set of sustainable
equilibrium adaptations to the Holocene may exist, but we have not yet
discovered them. Thus, the experiment is rather clean. The Pleistocene-Holocene
transition was a massive environmental change that was followed by more
or less unchanged environmental conditions for the last 11,500 years. In
response, human cultural evolution has generated a macroevolutionary trend
towards more efficient production per unit land area.
Given that competitive arms races drive the evolution of food production,
the problem is to discover what the rate-limiting steps are in the cultural
evolutionary processes leading to more intensive subsistence. The climate
change “experiment” considerably simplifies our search for causal explanations
by imposing a large, sudden, change and thereafter interfering minimally
with responses dictated by the competitive ratchet, rate limited by internal
processes. The mode of acquisition of agriculture (rarely indigenous development,
more commonly diffusion), rate of progress, and exact sequence of forms
of subsistence will depend upon local ecological and social conditions,
regional setting, historical happenstance, and the like. Thus, each example
of independent evolution of agriculture and each case of spread by conquest
or diffusion is a case that can be examined for clues as to the relative
importance of different evolutionary processes.
The prospects for getting data adequate to test hypotheses are good. Archaeologists,
in their attempts to explain particular transitions to plant rich and eventually
agricultural subsistence in particular locations typically offer scenarios
that are implicitly, at least, competitive ratchet models. Pristine origins
of agriculture require plants that are susceptible to improvement via domestication.
There have to be ways of incorporating proto-domesticates into the subsistence
systems of hunter-gatherers. Seasonality may be important in establishing
a premium on large scale planting for storage for the low productivity
season. If the local ecological conditions for intensification are favorable,
it occurs irreversibly. Workers such as MacNeish (1973), Flannery (1973),
and Harris (1977), inspired by a particular sequence of intensification
leading to agriculture, attempt to draw generalizations that should apply
to other sequences. As information has improved about the particular sequences,
a complex pattern of similarities and differences between cases has appeared.
MacNeish (1991) attempts to cover the diversity of patterns involved in
his “trilinear” theory of agricultural origins. Similarly, the spread of
agriculture from its original centers to more distant regions is susceptible
to archaeological, linguistic and biological investigations (Cavalli-Sforza,
et al. 1994). Studies of hunter-gatherer sequences of intensification that
stop far short of agriculture similarly often give us ecological and socially
detailed accounts of the intensification processes including some with
a quite clear account of the way the competitive ratchet probably worked
before agriculture proper (e.g. Bettinger and Baumhoff 1982).
Alternative hypotheses are weak
Aside from other forms of the climate change hypotheses described above,
archaeologists have proposed two prominent internal hypotheses, population
growth and cultural evolution, to explain the timing of the origin event.
They were formulated before the nature of the Pleistocene-Holocene transition
was understood, but are still the hypotheses most widely entertained by
archaeologists (MacNeish 1991). Neither hypothesis provides a close fit
with the empirical evidence.
Population Growth Has Wrong Time Scale
Cohen’s (1977) influential book argued that population pressure was responsible
for the origins of agriculture beginning at the 11,600 B.P. time horizon.
He imagines that subsistence intensification is driven by increases in
population density, and that a long, slow buildup of population gradually
drove people to intensify subsistence systems to relieve shortages caused
by population growth, eventually triggering a move to domesticates. Looked
at one way, this idea is just the population growth part of the competitive
ratchet. However, this argument fails to explain why pre-agricultural hunter-gatherer
intensification and the transition to agriculture began in numerous locations
after 11,600 years ago (Hayden 1995). Assuming that humans were essentially
modern by the Upper Paleolithic, they would have had 30,000 years to build
up a population necessary to generate pressures for intensification. Given
any reasonable estimate of the human intrinsic rate of natural increase
under hunting and gathering conditions (a large fraction of 1% yr-1
to 3% yr-1) populations substantially below carrying capacity
will double in a century or less. Even much smaller rates would be sufficient
to generate population pressure in far less than 30,000 years. The natural
time scale of demographic processes is far too short to explain the long
period of low population density in the Pleistocene followed by a rather
sudden, widespread interest in intensification of subsistence in a narrow,
rather recent, time horizon. It is also too rapid to explain the rather
gradual increase in the sophistication of agriculture and other production
systems over the last ten millennia.
Since
the population explanation for agriculture and other adaptive changes connected
with intensification remains very popular among archaeologists, it is worth
taking the time here to examine it formally. The logistic equation is one
simple, widely used model of the population growth. The rate of change
of population density, N, is given by:
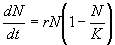
(1)
where
r is the “intrinsic rate of natural increase”—the rate of growth
of population density when there is no scarcity—and K is the “carrying
capacity,” the equilibrium population density when population growth is
halted by Malthusian checks. In the logistic equation, the level of population
pressure is given by the ratio N/K. When this ratio is equal to
zero the population grows at its maximum rate; there is no population pressure.
When the ratio is one, Malthusian checks prevent any population growth
at all. It is easy to solve this equation and calculate the length of time
necessary to achieve any level of population pressure, p = N/K.
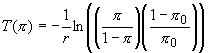
(2)
One
might think that this result is an artifact of the very simple model of
population growth. However, it easy to add much realism to the model without
any change of the basic result. Here we consider three such extensions:
more realistic population dynamics, a model with dispersal in space, and
a model in which people respond to population pressure by intensifying
subsistence.
More
realistic population dynamics. The logistic equation assumes that an
increment to population density has the same effect on population pressure
at low densities as at high densities. We know that this assumption is
not correct for all species. For example, hunters pursuing herd animals
that are easily spooked may generate much population pressure at low human
population densities because killing only a small fraction of the herd
make the many survivors difficult (but not impossible) to hunt. On the
other hand, subsistence farmers spreading into a uniform fertile plain
may feel little population pressure until all of plots of farmland of conveniently
workable size are taken. If returns to additional labor on shrinking farms
then drop steeply, most population pressure will felt at densities near
K. To allow for such effects, ecologists often utilize a generalized
logistic equation
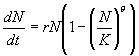
(3)
Population
pressure is now given by the term (N/K)q. If q > 1, population
pressure does not increase until densities approach carrying capacity,
as is usually the case for species like humans that have flexible behavior
and considerable mobility and thus can mitigate the effects of increasing
population density over some range of densities. It seems intuitive that
this would increase the length of time necessary to reach a given level
of population pressure. However, this intuition is wrong. The generalized
logistic can be used to derive a differential equation for p = (N/K)q
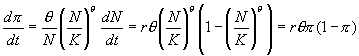
(4)
Thus,
the differential equation for population pressure is always the ordinary
logistic equation in which K = 1 and r is multiplied by q.
This means that when q > 1, it takes less time to reach a
given amount of population pressure than would be the case if q
= 1. Reduced population pressure at low densities leads to more rapid initial
population growth, and since population growth is exponential this more
than compensates for the fact that higher densities have to be reached
to achieve the same level of population pressure.
Allowing
for dispersal: Once, after listening to one of us propound this argument,
a skeptical archaeological colleague replied, “But you’ve got to fill up
all of Asia, first.” This natural intuitive response betrays a deep misunderstanding
of the time scales of exponential growth. Suppose that the initial population
of anatomically modern humans was only about 104 and
that the carrying capacity for hunter-gatherers is very optimistically
1 person per square kilometer. Given that the land area of the old world
is roughly 108 km2, p0 = 104/108
= 10–4. Then using equation 2 and again assuming r =
0.01, Eurasia will be filled to 99% of carrying capacity in about 1400
years. The difference between increasing population pressure by a factor
of 100 and by a factor of 10,000 is only about 500 years!
Moreover,
this calculation seriously over estimates the amount of time that
will pass before any segment of an expanding Eurasian population will experience
population pressure because populations will approach carrying capacity
locally long before the entire continent is filled with people. R. A. Fisher
analyzed the following partial differential equation that captures the
interaction between population growth and dispersal in space:
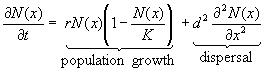
(5)
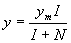
(6)
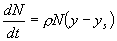
(7)
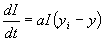
(8)
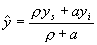
(9)
This picture of the interaction of demography and intensification leads
to predictions quite different from those of scholars like Cohen (1977).
For example, we do not expect to see any systematic evidence of increased
population pressure prior to major innovations (something that apparently
did not occur in the case of agricultural origins, Hayden 1995). If people
are motivated to intensify whenever population pressure causes per capita
income to fall below yi, and if, in the absence of intensification,
populations adjust relatively quickly to changes in K by growth
or contraction, then evidence of extraordinary stress, for example skeletal
evidence of malnutrition, is likely only when rapid environmental deterioration
exceeds a population’s capacity to respond via a combination of downward
population adjustment and intensification or other forms of innovation.
Some human populations might have curtailed birth rates in order to preserve
higher incomes at any given level of I. In a sense, such populations
have just redefined K to be a lower value that permits higher incomes
by employing what Malthus called the “preventative checks” on population
growth. The rest of the above analysis then applies with K measured
in suitably emic terms. Cultural differences in the value of ys
or K (Coale, 1986) will make evidence of stress more likely in populations
where the effective carrying capacity is close to the subsistence carrying
capacity compared to populations that reduce population growth rates some
ways from absolute subsistence limits set by preventative checks. Similarly,
populations that begin to intensify at a relatively high value of yi,
will be less likely to suffer in environmental crunches. In other words,
population pressure will tend to stay constant to the extent that rates
of population growth and intensification are successful in adjusting subsistence
to current conditions. Normally population growth and decline are quite
rapid processes relative to rates of innovation and will keep average population
size quite close to K. Short-term departures from K caused
by short-term environmental shocks and windfalls should be the commonest
reasons to see especially stressed or unstressed populations.
Thus, for parameter values that seem anywhere near reasonable to us, population
growth on millennial time scales will be rate limited by rates of improvement
in subsistence efficiency not by the potential of populations to grow,
just as Malthus argued. Populations can behave in non-Malthusian ways only
under extreme assumptions about population dynamics. If the rate of intensification
is more rapid than exponential population growth for any significant time
period, then per capita incomes can rise under a regime of very rapid population
growth, as in the last few centuries. This regime, if it had occurred in
the past, should be quite visible in the historical and archaeological
record because it so rapidly leads to large populations. Alternatively,
population growth may have been limited in past populations by the analog
of the modern demographic transition. Thus, hunter-gatherers might have
resisted the adoption of plant based intensification because they viewed
the life style associated with plant collecting or planting as a decrement
to their incomes. However, resisting intensifications that increase human
densities makes such groups vulnerable to competitive displacement by the
intensifiers unless the greater wealth of the population limiters allows
them to successfully defend their resource rich territories. On the evidence
of the fairly rapid rate of spread of intensified strategies once invented,
such defense is seldom successful (e.g. Ammerman and Cavalli-Sforza 1971;
Bettinger and Baumhoff 1982).
Of course, in a time as variable as the Pleistocene, populations may well
have spent considerable time both far above and far below instantaneous
carrying capacity. If agricultural technology were quick and easy to develop,
the population pressure argument would lead us to expect Pleistocene populations
to shift in and out of agriculture and other intensive strategies as they
find themselves in subsistence crises due to environmental deterioration
or in periods of plenty due to amelioration. Most likely, minor intensifications
and de-intensifications were standard operating procedure in Pleistocene
times. However, the time needed to progress much toward plant rich strategies
was greater than the fluctuating climate allowed, especially given CO2
limited plant production.
Cultural Evolution Has Wrong Time Scale
Robert Braidwood (1960) argued a “settling in” hypothesis to the effect
that once humans acquired enough familiarity with plant resources, they
naturally turned to them as a more efficient source of calories. This proposal
is also quite consistent with the competitive ratchet. The issue is when
the settling in process began. If our argument is correct, settling in
could only begin in the latest Pleistocene and at the beginning of the
Holocene. On this interpretation, our argument is an elaboration of Braidwood’s
insight that cultural evolution is a rather slow process. However, if we
interpret his argument to be that the settling in process began with the
evolution of behaviorally modern humans, the time scale is wrong again.
There is no evidence that people were making any progress at all toward
agriculture for 30,000 years, and Braidwood’s excavations at Jarmo show
that more like 4,000 years was enough to go from a plant-light hunting
and gathering subsistence system to settled village agriculture in a fast
case. 10,000 years in the Holocene was sufficient for even the slowest
cases to develop plant-intensive gathering technologies.
Strong Similarities and Differences in Different Replicates of the Experiment
The first test of the general hypothesis outlined here is whether the great
mass of human societies have indeed been on an out-of-equilibrium trajectory
toward more intensive subsistence techniques for the last 15,000 years
(especially for the last 11,600 years). Most evolutionary scenarios imply
quite different patterns. For example, if the Pleistocene-Holocene transition
played a small role in creating environmental conditions favoring agriculture,
then subsistence innovations should not be correlated with its appearance.
If there has been no recent external change of global proportions, there
is no reason to expect any coherent progressive trends among all the world’s
societies.
Agriculture was independently invented ~ 10 times in the Holocene
Table 1 gives a rough time line for the origin agriculture in seven fairly
well understood centers of domestication, two more controversial centers,
two areas that acquired agriculture by diffusion, and two areas that were
without agriculture until European conquest. The list of independent centers
is complete as far as current evidence goes, and while new centers are
not unexpected it is hard to believe that the present list will be doubled
by further investigation. The areas that acquired agriculture by diffusion
are very numerous, so the three areas in Table 1 are but a small sample.
The number of non-arctic areas without agriculture at European contact
is small and the two listed, Western North America and Australia, are the
largest and best known.
Archaeologists are convinced that the seven centers of domestication are
indeed independent on several grounds. First, the domesticates taken up
in each center are distinctive and no evidence of domesticates from other
centers turns up early in the sequence. For example, the Eastern North
American center took into cultivation sunflower, a goosefoot, marsh elder,
a squash, and other local plants. Long after these plants were taken into
cultivation, maize was traded into the region and was grown in small quantities
beginning around 2,000 B.P. However, it remained a minor domesticate until
around 1,100 B.P., when it suddenly became the dominant domesticate, crowding
out several traditional cultivars (Smith 1989). The Eastern squash is closely
related to the Mexican squash, but genetic and morphological evidence indicates
that two subspecies were independently taken into cultivation. Second,
archaeology suggests that none of the centers had agricultural neighbors
at the time that their initial domestications were undertaken. The two
problematic centers, New Guinea and Lowland South America, present difficult
archaeological problems (Smith 1995). Sites are hard to find and organic
remains are rarely preserved. The New Guinea evidence consists of apparently
human constructed ditches that might have been used in controlling water
for taro cultivation. The absence of documented living sites associated
with these features makes their interpretation quite difficult. The Lowland
South American evidence consists of starch grains embedded in pottery fragments
and phytoliths, microscopic silicious structural constituents of plant
cell walls. The large size of some early starch grains and phytoliths convinces
some archaeologists that root crops were brought under cultivation in the
Amazon Basin at very early dates. Given the recency with which the Eastern
North American and North and South China centers were firmly established,
the discovery of a few more centers is likely. Recall Ehret’s (1998) linguistic
evidence for multiple centers in Africa.
Note that the date of the initiation of agriculture varies quite widely.
In the Near East, Natufian peoples lived in settled villages and exploited
the wild ancestors of wheat, barley beginning in the Allerød-Bølling
warm period (14,500 -12,900 B.P.) (Henry 1989), and then reverted to mobile
hunting and gathering during the sharp, short Younger Dryas (12,500-11,500
B.P.), the last of the high-amplitude fluctuations that were characteristic
of the last glacial (Goring-Morris and Belfer-Cohen 1997; Bar-Yosef and
Meadow 1995). Ice core evidence suggests that the Younger Dryas was significantly
colder and more variable than the Allerød-Bølling and the
Holocene (von Grafenstein, et al. 1999; Mayewski, et al. 1993). Post-Natufian
cultures began to domesticate the same species virtually the moment warm
and stable conditions returned after the Younger Dryas, around 11,600 B.P.
Unfortunately, a flat spot in the 14C/calendar year calibration
curve makes precise dating difficult for the most critical several hundred
years centered on 11,600 B.P. (e.g., Fiedel 1999). In North, and possibly
South, China, however, agriculture probably followed within a thousand
years even though the earliest clearly agricultural complexes are considerably
later (Crawford 1992; An 1991). Agriculture may prove to be as early in
North China as in the Near East, since the earliest dated sites, which
extend back to 8000 B.P., represent advanced agricultural systems that
must have taken some time to develop. Excavations in North China north
of the earliest dated agricultural sites document a technological change
around 11,600 B.P. signaling a shift toward intensive plant and animal
procurement that may have set this process in motion (Elston, et al. 1997).
The
dates in Table 1 reflect considerable recent revision stemming from accelerator
mass spectrometry 14C dating, which permits the use of very
small carbon samples and can be applied directly to carbonized seeds and
other plant parts showing morphological changes associated with domestication.
Isolated seeds tend to work their way deep into archaeological deposits,
and dates based on associated large carbon samples (usually charcoal) often
gave anomalously early dates.
The exact sequence of events also varies quite widely. For example, in
the Near East, sedentarism preceded agriculture, at least in the Levantine
Natufian sequence, but in Mesoamerica crops seem to have been added to
a hunting and gathering system that was dispersed and rather mobile (MacNeish
1991: 27-29). For example, squash seems to have been cultivated around
by 10,000 B.P. in Mesoamerica, some 4,000 years before corn and bean domestication
began to lead to the origin of a fully agricultural subsistence system
(Smith 1997). Some mainly hunting and gathering societies seem to have
incorporated small amounts of domesticated plant foods into their subsistence
system without this leading to full scale agriculture for a very long time.
According to MacNeish, the path forward through the whole intensification
sequence varied considerably from case to case.
Intensification of Plant Gathering Precedes Agriculture
In all known cases, the independent centers of domestication show a sequence
beginning with a shift from a hunter-gatherer subsistence system based
disproportionately upon the capture of large game to a strategy based upon
small game and especially plant seeds or other labor-intensive plant resources
(Hayden 1995). The reasons for this shift are the subject of much work
among archaeologists (Bettinger in press). These shifts invariably occur
in the latest Pleistocene or later. Driven by the competitive ratchet,
hunter-gathers who subsidize the hunting population with a large measure
of plant-derived calories will tend to deplete the most desirable big game
to levels that cannot sustain hunting specialists. Once better climates
made the shift possible, the first escalation began in what would become
the agricultural subsistence race. Braidwood’s reasoning that pioneering
agriculturalists would have gained their intimate familiarity with proto-domesticates
first as gatherers is logical and supported by the archaeology.
The cases where intensification of plant gathering did not lead to agriculture
are in some ways as interesting as the cases in which it did. The Jomon
of Japan represents one extreme (Imamura 1996). Widespread use of simple
pottery, a sure marker of well developed agricultural subsistence in Western
Asia, was very early in the Jomon, contemporary with the latest Pleistocene
Natufian in the Near East. By 11,000 yrs B.P., the Jomon people lived in
settled villages, depended substantially upon plant foods, and used massive
amounts of pottery. However, the Jomon domesticated no plants, although
they cultivated a few minor crops like bottle gourds. Agriculture came
to Japan with imported rice from the mainland only about 2,500 B.P. Interestingly,
acorns were a major item of Jomon subsistence. The people of California
were another group of sedentary hunter-gatherers that depended heavily
on acorns. However, in California the transition to high plant dependence
began much later than in the Jomon (Wohlgemuth 1996). Millingstones for
grinding small seeds became important after 4,500 B.P., although seeds
were of relatively minor importance. After 2,800 B.P. acorns processed
with mortars and pestles became an important subsistence component and
small seeds faded in comparative importance. In the latest period, after
1200 B.P., quantities of small seeds were increasingly added back into
the subsistence mix alongside acorns in a plant dominated diet. Other peoples
with a late onset of intensification include the Australians. The totality
of cases tell us that any stage of the intensification sequence can be
stretched or compressed by several thousand years even though reversals
are rare (Harris 1996; Price and Gebauer 1995). Farming gave way to hunting
and gathering in the southern and eastern Great Basin of North America
after a brief extension of farming into the region around 1,000 B.P.(Lindsay
1986). A similar reversal occurred in southern Sweden between 2,400 and
1,800 B.P., Zvelebil 1996), and we have already noted the case of Polynesian
de-intensification on newly settled islands.
More Intensive Technologies Tend to Spread
Once well-established agricultural systems exist, they expand at the expense
of hunting and gathering neighbors (Bellwood 1996). Ammerman and Cavalli-Sforza
(1971) summarize the movement of agriculture from the Near East to Europe,
North Africa and Asia. The spread into Europe is best documented. Agriculture
reached the Atlantic seaboard about 6,000 B.P. or about 4,000 years after
its origins in the Near East. The regularity of the spread, and the degree
to which it was largely a cultural diffusion process as opposed to a population
dispersion as well, are matters of debate. Cavalli-Sforza et al. (1994:
296-299) argue that demic expansion by Western Asians was an important
process with the front of genes moving at about half the rate of agriculture.
They imagine that pioneering agricultural populations moved into territories
occupied by hunter-gatherers, intermarried with the pre-existing population.
The then mixed population in turn sent agricultural pioneers still deeper
into Europe. They also suppose that the rate of spread was fairly steady,
though clearly frontiers between hunter-gathers and agriculturalists stabilized
in some places (Denmark, Spain) for relatively prolonged periods. Zvelebil
(1996) stresses the durability of frontiers between farmers and hunter-gatherers
and the likelihood that in many places the diffusion of both genes and
ideas about cultivation was a prolonged process of exchange across a comparatively
stable ethnic and economic frontier. Further archaeological and paleo-genetic
investigations will no doubt gradually resolve these debates. Clearly,
the spread process is at least somewhat heterogeneous.
Other examples of the diffusion of agriculture are relatively well documented.
For example, maize domestication is dated to about 6200 B.P. in Central
Mexico and to about 4000 B.P. in , in the southwestern U.S. (New Mexico;
Smith 1995; Matson 1999). In this case, the frontier of maize agriculture
stabilized for a long time, only reaching the Eastern U.S. at a comparatively
late date as noted above. Maize failed entirely to diffuse westward into
the Mediterranean parts of California even though peoples growing it in
the more arid parts of its range in the Southwest used irrigation techniques
that would have worked well there. As with the origin process, the rate
of spread of agriculture exhibits an interesting degree of variation.
Agriculture a “Natural” Outcome of More Intensive Technologies
A number of plant and animal biologists have taken an interest in the process
of the evolution of domesticates (Zohary and Hopf 1993, Rindos 1984, Bretting
1990, Smith, 1995). The first changes in seed crop plants generally include
larger seeds, the loss of natural seed dispersal mechanisms, and the loss
of seed dormancy. These changes do not necessarily require any conscious
selection by cultivators. Once humans began to intensively exploit wild
seed plants, especially once they prepared seedbeds and deliberately planted
seeds, these changes will tend to be automatic. Larger seeds yield more
competitive seedlings in the artificial seedbed. Those seeds that do not
drop off the stalk are more likely to be harvested, favoring genotypes
with non-shattering seed heads. With humans controlling the time of planting
and storing seed in cool, dry locations, seed dormancy, rather than producing
adaptively facultative germination, merely increases the chance that a
seed will fail to germinate in time to be harvested. As Flannery (1973)
observed, it is not clear which species is the domesticated and which the
domesticator in agriculture! Some plants responded to human harvest in
such a way that they induced humans to plant more of that species in competition
with others, raise larger numbers of children to plant still more fields,
and eventually to carry the plant across continents.
Of course, every human population includes close observers and inveterate
experimenters. No doubt deliberate ingenuity and sustained artificial selection
also played roles in the evolution of domesticates, as they do in the development
and conservation of landraces today (Brush 1995).
What Regulates Tempo and Mode of Cultural Evolution?
The overall pattern of subsistence intensification is clearly consistent
with the hypothesis that agriculture was impossible in the Pleistocene
but mandatory in the Holocene, but the real test is whether or not we can
give a satisfactory account of the variation in the rate and sequence of
intensification. Work on this project is in its infancy, and only some
rather preliminary and speculative answers are possible.
External Processes Play a Role
In
general, external processes of macroevolution will tend to operate at longer
time scales and internal processes at shorter time scales. Evolution has
to be flexible and rapid enough to roughly track environmental variation,
and environmental variation is greater at longer than shorter time scales.
Those species that fail to track longer-term variation will go extinct,
and will not contribute to the evolutionary processes we see in action.
At the same time, no evolutionary process responds instantaneously. There
is always stickiness at short enough time scales. If the world were simple,
the time scales of internal and external processes would be entirely separate.
The Pleistocene/Holocene transition, we argue, is a good natural experiment
because the separation of scales is very good. To a first approximation,
the lack of agriculture before the Holocene is due to the external effect
of the Pleistocene climate; after that the rate of progress toward ever
more intensive subsistence is largely regulated by internal processes.
However, external causes did not entirely disappear in the Holocene. To
completely isolate the effects of internal processes we have to control
for residual external effects.
Climate change may play a small role. The Holocene climate is only
invariant relative to the wild oscillations of the last glacial (Lamb,
1977). For example, seasonality (difference between summer and winter insolation)
was at a maximum at the beginning of the Holocene and has fallen since.
The so-called “Climatic Optimum,” a broad period of warmer temperatures
during the middle Holocene, caused a wetter Sahara, and the expansion of
early pastoralism into what is now forbidding desert. The late medieval
onset of the Little Ice Age caused the extinction of the Greenland Norse
colony. Agriculture at marginal altitudes in places like the Andes seems
to respond to Holocene climatic fluctuation (Kent 1987). While the effect
of Holocene climate fluctuations on regional sequences must always be kept
in mind, the dominance of the nearly always monotonic tendency to increased
subsistence efficiency per unit land seems likely to be driven by other
processes.
Geography may play a big role. Diamond (1997) argues that Eurasia has
had the fastest rates of cultural evolution in the Holocene because of
its size and to a lesser extent its orientation. Plausibly, the number
of innovations that occur in a population is, to a first approximation,
a function of total population size and the flow of ideas between sub-populations.
Societies acquire most innovations by diffusion from other societies, and
isolated societies will be handicapped in their rate of innovation by a
slower rate of diffusion. Eurasia is the largest continent and is especially
large in its east-west dimension. Innovations occurring at one end of the
continent will eventually diffuse to the other, spreading along lines of
latitude with relatively similar environments. Small land-masses like Australia
and New Guinea are substantially isolated from the larger world and have
a much smaller base of innovators. The Americas, though quite respectable
in size, are oriented with their major axis north-south. Consequently innovations
have to mainly spread across lines of latitude from the homeland environment
to quite different ones. Wheat varieties originating in the Near East could
spread to Spain and to Western China without substantial modification.
The maize moving north from Mexico would have to be selected to respond
appropriately to longer day length and a shorter growing season. Maize
spreading to South America would have to move through a belt of hot, wet,
tropics with little day length variation before reaching the cool, semiarid,
subtropical highlands of South America so similar to its original homeland.
Since we know that the original centers of agricultural innovation were
relatively small compared to the areas to which agriculture later spread,
it is clear that complex adaptations arise infrequently, but are relatively
easy to acquire by imitation. The most isolated agricultural region in
the world, Highland New Guinea, underwent an economic revolution in the
last few centuries with the advent of American sweet potatoes, a crop that
thrives in the cooler highlands above the malaria belt of lowland New Guinea
(Wiessner and Tumu 1998). The agriculture of both the Old and New Worlds
was impacted substantially by the Columbian exchanges beginning 500 years
ago. Eurasian agriculture found good uses for American maize, potatoes,
squash, beans, peppers, and tomatoes, despite the already deep list of
ancient Eurasian cultivars. The marginal benefit of increased numbers,
hence diversity, of domesticates and inventors seems to have been substantial
even on the largest continent on earth.
Coevolutionary Processes Play a Big Role
In the case of domestication, the distinction between external and internal
regulation of process rates is ambiguous without further definition. The
genetic changes in the proto-domesticate, as it responds to cultural practices,
are external to the cultural evolutionary system in one sense. In another,
the coevolutionary system as a whole is, perhaps, more naturally seen as
having intertwined the evolution of human culture and plant and animal
genomes as to make such a distinction artificial. Human genomes may also
be involved in the coevolutionary system.
Agriculture
requires pre-adapted plants and animals. In each center of domestication,
people domesticated only a handful of the wild plants that they formerly
collected, and of this handful even fewer are widely adopted outside those
centers. The same is true for domesticated livestock. Wheat, rice, and
maize make disproportionate contributions to total world crop production.
Cattle, sheep, goats, hogs, and chickens make an outsized contribution
to total world livestock production. A tiny fraction of the world’s plant
and animal diversity ended up as significant domesticates. Zohary and Hopf
(1989) have listed some of the desirable features in plant domesticates.
Aside from obvious things like large seed size, most Near Eastern domesticates
had high rates of self-fertilization. This means that farmers can select
desirable varieties and propagate them with little danger of gene flow
from other varieties or from weedy relatives. Maize, by contrast, outcrosses
at high rates. Perhaps the later and slower evolution of maize compared
to Near Eastern domesticates is due to the difficulty of generating responses
to selection in the face of gene flow from unselected populations. Smith
(1995) discusses the many constraints on potential animal domesticates.
For example, many large ungulates like deer depend upon rapid flight to
avoid predators. They are thus very skittish and adapt poorly to human
handling. Larger herd ungulates that are less susceptible to large predators,
like cattle, have shorter flight distances and more stolid temperaments.
Even in the most favorable cases, the evolution of new domesticates is
not an instantaneous process. In at least some times and some places, the
rate of evolution of domesticates was likely the rate-limiting step in
the agricultural intensification process.
Diamond (1997), drawing on the work of Blumler (1992), notes that the Near
Eastern region has a flora that is unusually rich in large-seeded grasses
(Table 2). California, by contrast, is quite depauperate in large seeded
grasses, having not a single species that passed Blumler’s criterion. California
has so many climatic, topographic, and ecological parallels with the precocious
Fertile Crescent that its very tardy development of plant-intensive subsistence
systems is a considerable puzzle. The presence of a large suite of agriculturally
preadapted plants in the Near East but not in California provides a plausible
hypothesis to explain the difference in agriculture but not plant intensification
itself.
Humans
have to adapt biologically to agricultural environments. While the transition
from hunting and gathering to agriculture resulted in no genetic revolution
in humans, a number of modest new biological adaptations were likely involved
in becoming farmers. Perhaps the best-documented case is the evolution
of adult lactose absorption in human populations with long histories of
dairying (Durham, 1991). Most human populations, as in mammals more generally,
adults lose the ability to digest the milk sugar lactose. However, the
frequency of adult absorption reaches near fixation in Western European
and African populations with a long tradition of drinking fluid milk. It
is present in intermediate frequencies in populations in the circum-Mediterranean
and other subtropical regions that more typically consume milk as cheese
or fermented products low in lactose. Other cases suggest human genetic
coevolution with domestication. For example, agricultural populations metabolize
alcohol more rapidly than hunter-gatherers, either to improve the nutritional
yield from such products or to avoid toxic effects. To some extent the
relatively slow rate of biological adaptation will act as a drag on the
rate of cultural innovation.
Diseases limit population expansions, protect inter-regional diversity.
McNeill (1979) and Crosby (1986) draw our attention to the coevolution
of people and diseases. The increases in population density that resulted
from the intensification of subsistence invited the evolution of epidemic
diseases that could not spread at lower population densities. One result
of this process was likely to slow population growth and hence slow the
rate at which growing populations fuel the competitive ratchet. In more
disease prone environments, such as malarial tropical lowlands, this restraint
on the growth process may have been quite severe. A suite of hemoglobins
have arisen in different parts of the world that confer partial protection
against malarial parasitism (Cavalli-Sforza, et al. 1994). Some authors
have suggested that land clearance associated with agriculture increased
malarial infection rates. Cavalli-Sforza et al. estimate that it would
take about 2,000 years for a new mutant hemoglobin variant to reach equilibrium
in a population of 50,000 or so individuals. The relatively late and slow
rate of the evolution of agricultural systems in Africa might, in part
be attributable to the high rates of disease infection of people and livestock
slowing the rate of intensification induced population growth to the macroevolutionary
time scale (see also Gifford-Gonzales in press).
As both McNeill and Crosby note, regional populations tend to have their
own diseases, to which they are more or less immune, while they tend to
be susceptible to the diseases of people from other regions. This pattern
of susceptibility will tend to act against communication between regions.
Travelling strangers who might bring new ideas and stronger competition
will tend to fall sick and to bring diseases that cause their hosts to
fall sick (at least temporarily reducing competition for land). Disease
barriers will tend to isolate regions and hence slow rates of diffusion
of ideas. This effect was probably historically most severe for Africa
and other Old World tropical areas that were quite unhealthy for people
from temperate Eurasia. On the other hand, in cases like the European invasion
of the New World and smaller insular areas with low loads of infectious
disease, European conquest was greatly aided if not entirely made possible
by a disease gradient operating at the expense of native populations. To
the extent that populations sustaining higher densities sustain more endemic
diseases, they will have a competitive edge against less dense populations
without a history of exposure to so many diseases.
Purely Internal Processes Play a Role
The macroevolutionary patterns in which we are most interested are the
processes strictly internal to cultural evolution that might limit the
rate of intensification. We (Boyd and Richerson 1985; Bettinger 1991) view
cultural evolution as a Darwinian process of descent with modification.
Evidence about the macroevolutionary tempo and mode of cultural evolution
bear directly on our picture of the micro scale processes that form the
body of the theory. For many characters human decisions have relatively
weak effects in the short run although they can be powerful when integrated
over many people and appreciable spans of time. If the rational-choice
element in our models is sufficiently strong, then the rate of cultural
evolution itself will never be rate limiting and cultural evolution will
lack the Darwinian element. The tempo of subsistence intensification will
then be entirely governed by external or coevolutionary processes.
Selective processes can also cause quite rapid evolution compared to the
time scales of external processes. Darwinians use the metaphor of an “adaptive
topography” to picture the action of natural selection. Selection acts
to drive populations up slopes of fitness toward peaks in an adaptive topography.
If fitness topographies are smooth, the populations climb more or less
directly and quickly to global optima. On the other hand, if fitness topographies
are rough, much more complex trajectories will ensue. Populations will
tend to get stuck on local peaks in the foothills of a fitness topography.
Chance events may eventually carry small populations across fitness valleys.
For example, minor climate change may shift the topography allowing one
population to escape a local peak and run up to a higher one. A population
of populations (a metapopulation in the jargon) will, by various historically
contingent routes, gradually filter to higher and higher fitness peaks.
Historically minded scholars have always believed that such complexities
were important (e.g. Vayda 1995), but only recently have models of biological,
cultural, and economic change become sophisticated enough to investigate
them formally. Typically the structural changes in such models are straightforward
extensions of classical models that behave ahistorically. We think that
such models should lead to closer and more productive interactions between
theorists and historians (Boyd and Richerson 1992). Several internal processes
may lead to rough fitness topographies and thus act to limit the rate of
cultural evolution of intensification.
New technological complexes evolve with difficulty. One problem that
will tend to slow the rate of cultural (and organic) evolution is the sheer
complexity of adaptive design problems. As engineers have discovered when
studying the design of complex functional systems, discovering optimal
designs is quite difficult. Blind search algorithms often get stuck on
local optima. Piecemeal improvements at the margin are not guaranteed to
find globally optimal adaptations by myopic search. Yet, myopic searches
are what Darwinian processes do.
Parallel problems are probably rife in human subsistence systems. The shift
to plant-rich diets is complicated because plant foods are typically deficient
in essential amino acids, and vitamins, have toxic compounds to protect
them from herbivore attack, and are labor intensive to prepare. The diet
of Pleistocene hunters and gathers probably focused on high rates of meat
intake supplemented by high quality plant foods such as ripe fruit and
nuts. High quality plant resources are scarce and the inefficiency of natural
herbivore populations means that meat offtake rates are usually quite limited.
Intensification requires a focus on seeds low in essential amino acids
(maize), tubers with poisonous protection (bitter manioc), and the like.
Even the best plant resources like wheat require protein supplementation
with animal products or legumes. Skeletal material suggests that early
agricultural peoples were often less well nourished than their hunter-gather
ancestors (Cohen and Armelagos 1984). Finding a mix of plant and animal
foods that provides adequate diet is not a trivial problem. Many different
mixes may work more or less well, but which one(s) are globally or nearly
globally optimal?
New World farmers eventually discovered that boiling maize in wood ashes
improved its nutritional value. The hot alkaline solution breaks down an
otherwise indigestible seed coat protein that contains some lysine, an
amino acid that is low in maize relative to human requirements (Katz, et
al. 1974). Hominy and masa harina, the corn flour used to make tortillas,
are forms of alkali treated maize. The value of this practice could not
have been obvious to its inventors or later adopters, yet all American
populations that made heavy use of maize employed it. The dates of origin
and spread of alkali cooking are not known. It has not been reinvented
in Africa even though many African populations have used maize for centuries.
The prehistory of subsistence intensification consists of a long sequence
of inventions that gradually increase the sophistication of food producing
systems. Arboriculture, irrigation, animal traction, dairying, wool use,
and the like were added to toolkits thousands of years after people were
committed to agricultural production. Plausibly, each of these inventions
was the product of prolonged experimentation and development in a center
of origin, followed by a slow spread to adjacent areas. Once again, the
large and size east-west axis of the Eurasian land mass likely accelerated
the pace of development relative to other areas. The New World, among other
problems, was poor in animal species suitable for domestication due to
the terminal Pleistocene megafaunal extinction event, arguably caused by
the initial wave of human settlers (Martin and Klein 1984). To make matters
worse, the most important New World domesticates, the camelids of the temperate
Andes, did not spread across the wet tropical belt into Central and North
America. As intensification proceeded, each innovation adopted had to be
fitted into an existing system of land use, labor allocation, and diet.
In rapidly developing Eurasia, the rate of subsistence intensification
could well have been limited partially or even mainly by the technical
complexity of the innovation process.
Some of the difficulties in evolving new subsistence systems may stem from
dynamic problems in the economy. Lee (1986) imagines that the rate of innovation
is correlated with the rate of profit for invested capital. In a Ricardian
world, profits will be low when population density is low because wages
will be too high. Conversely, when population density is too high, profits
will be squeezed by rents to land. With profits low, entrepreneurs will
lack surplus capital to invest in innovation. Entrepreneurs will thus innovate
only in a narrow window between these two constraints. If we imagine a
population fluctuating due to epidemic disease, famine, and the like, little
bursts of innovation will occur as population fluctuates through the key
window. Day and Walter (1989) investigate a similar model the exhibits
a chaotic growth path.
New social institutions evolve with difficulty. As anthropologists
and sociologists such as Julian Steward (1955) have long emphasized, human
economies are social economies. Even in the simplest human societies, hunting
and gathering is never a solitary occupation. At the minimum, such societies
have division of labor between men and women. Hunting is typically a cooperative
venture. The unpredictable nature of hunting returns typically favors risk
sharing at the level of bands composed of a few cooperating families because
most hunters are successful only every week or so (Winterhalder 1986).
Portions of kills are distributed widely, sometimes exactly equally, among
band members.
The deployment of new technology requires changes in social institutions
to make best use of innovations. The increasing scale of social institutions
associated with rising population densities during the Holocene have dramatically
reshaped human social life. Even the first steps of intensification required
significant social changes. Gathering is generally the province of women
and hunting of men. Male prestige systems are generally tied up with hunting
success. A shift to plant resources requires scheduling activities around
women’s work rather than men’s. Using more plants will conflict with men’s
preferences as driven by a desire for hunting success; it will require
a certain degree of women’s liberation to intensify subsistence. Since
men generally dominate women in group decision-making, male chauvinism
will tend to limit intensification. Bettinger and Baumhoff (1982) argue
that the spread of Numic speakers across the Great Basin a few hundred
years ago was the result of the development of a plant-intensive subsistence
system in the Owens Valley. Once developed, the spread eastward to the
Rockies because the Numics could outcompete the previous inhabitants who
neglected plant resources in favor of the hunt. The evidence suggests a
demic expansion rather than a cultural diffusion. Apparently, the groups
specialized in the hunt would not or could not shift to the more productive
economy to defend themselves, perhaps because males clung to the outmoded,
plant poor, subsistence. Those with a vested interest in current social
institutions are naturally conservative.
Classic hunting and gathering societies are relatively small in scale,
and are quite egalitarian (Woodburn 1980). Hence they have very informal
leadership. The evolution of formal coercive leadership, hierarchical command
and control systems, and the rise of marked social inequality are resented
and resisted, most likely because they violate our social “instincts,”
for example our sense of what is and is not fair (Richerson and Boyd 1998,
1999). As people began to collect in villages, ethnographic analogies suggest
that formal political leaders likely became important. As the agricultural
toolkit expanded, larger markets for sophisticated products came to favor
an ever-finer division of labor. Various sorts of institutions arose to
manage the division of labor—caste systems, command economies, market systems,
international trade systems and the like. The creation of storable and
portable wealth exacerbates defense and security problems, leading to the
rise of armies and police forces.
The evolution of complex social institutions is arguably as or more difficult
than the evolution of technological innovations. If so, social institutions
will tend to regulate technological progress. For example, North and Thomas
(1973) argue that new and better systems of property rights set off the
modern industrial revolution, much as Bettinger and Baumhoff argue that
new social institutions were required to favor innovations in plant collection
strategies at the inception of the intensification trajectory. A major
revolution in property rights is likely also necessary for intensive hunting
and gathering and agriculture to occur (Bettinger 1999). The sharing ethic
that characterizes many simple hunter-gatherer groups discourages individual
efforts to intensify production since freeloaders enjoy the extra resources
generated by hard working individuals. Thus, accumulating resource surpluses
to store for seasons of shortfall is heavily contingent on an ideology
in which collected resources are regarded as private property. The shift
from resources as public goods to resources as private goods, however,
is likely to be difficult since any form of innovation along these lines
will, at least initially, be regarded as antisocial. The comparative history
of the social institutions of intensifying societies exhibits many examples
of societies getting far ahead of others in one dimension or another. For
example, the Chinese merit based bureaucratic system of government was
established at the expense of the landed aristocracy, beginning in the
Han dynasty (2,200 B.P.) and completed in the Tang (1,400 B.P.) (Fairbank
1992). In the West similar institutions arose only in the last few centuries.
Progress aside, functionally analogous institutions are notoriously different
in different culture areas. The Indian caste system is a unique, or at
least uniquely hypertrophied, method of organizing a complex division of
labor. The Turkish system of raising armies (Janissaries) and even ruling
classes (Mamlukes) by enslaving Christian boys is similarly unique. Social
institutions seem generally to diffuse much less readily than technology.
In the late mediaeval and early modern period, the West acquired a considerable
number of important technical innovations from China, but certainly not
the idea of a Confucian bureaucracy. Social institutions violate four of
the conditions that tend to facilitate diffusion (Rogers 1983). Foreign
social institutions are often (i) not compatible with existing institutions,
(ii) complex, (iii) difficult to observe, and (iv) difficult to try out
on a small scale. Technical innovations like the compass and the cannon
are thus much more easily diffused than social institutions. Hence historical
differences in social organization, many of them less nearly optimal than
those already traditional elsewhere, are quite persistent.
Ideology may play a role. Forces other than strictly utilitarian ones
like natural selection govern the evolution of fads, fashion, and belief
systems. They are susceptible feedback and runaway dynamics that defy common
sense (Boyd and Richerson 1985: Chap. 8). The arbitrariness of the runaway
dynamic produces path dependent historical change, often in defiance of
economic or fitness rationality. The links between belief systems and subsistence
are nevertheless incontestably strong. To build a cathedral requires an
economy that produces surpluses that can be devoted to grand gestures on
the part of the faithful. The moral precepts inculcated by the clergy in
the cathedral underpin the institutions that in turn regulate the economy.
Arguably, ideological innovations often drive economic change. Recall Max
Weber’s classical argument about the role of Calvinism in the rise of capitalism.
The Twentieth Century’s largely failed experiments with nationalist and
socialist command economies is an example of an ideology driven exploration
in the space of all possible social formations, but not one that was very
well controlled to make progress. Contemporary Russia
illustrates the great difficulty of making the transition from a failed
set of institutions to a new set. So, arguably, does the inability of the
United States to take risks with economic growth in pursuit of policies
that may be necessary to prevent global warming and similar threats to
economic sustainability. Contemporary consumerism is arguably the result
of institutionalizing the pathological tendency of status competition to
escalate without limit (Frank and Cook 1995, Easterlin 1995).
We Have a Shadowy Outline of the Tempo and Mode of Cultural Evolution
The large step change in environment at the Pleistocene-Holocene transition
set off the trend of subsistence intensification of which modern industrial
innovations are the latest examples. The diversity of trajectories taken
by the various regional human sub-populations since 11,600 B.P. are natural
experiments that reveal the internal limitations on the rate of cultural
evolution. We believe that the discussion above is not only a basic review
of the main cultural macroevolutionary hypotheses currently entertained
by scholars but also a crude picture of how cultural macroevolution has
actually happened. There is a rather long list of processes that probably
interacted to regulate the nearly unidirectional trajectory of subsistence
intensification, population growth, and institutional change that the world’s
societies have followed in the Holocene. Social scientists are in the habit
of treating these processes as mutually exclusive hypotheses. They seem
to us to be competing but certainly not mutually exclusive. At the level
of qualitative empiricism, tossing any one out entirely leaves puzzles
that are hard to account for and produces an obvious caricature of the
actual record of change. If this conclusion is correct, the task for historically
minded social scientists is to refine estimates of the rates of change
that are possible due to the various evolutionary processes and to estimate
of how those rates change as a function of natural and socio-cultural circumstances.
The origins of agriculture and ensuing events are an excellent macroevolutionary
“laboratory” for understanding the internal processes of cultural evolution.
The End of Agriculture?
Those who are familiar with the Pleistocene often remark that the Holocene
is just the “present interglacial.” The return of climate variation on
the scale that characterized the last glacial is quite likely if current
ideas about the Milankovich driving forces of the Pleistocene are correct.
Sustaining agriculture under conditions of much higher high frequency environmental
variation than farmers currently cope with would be a very considerable
technical challenge. At the very best, lower CO2 concentrations
and lower world average precipitation suggest that world average agricultural
output would fall considerably.
In one sense, though, the Holocene is not just another interglacial. Recall
that Petit et al. (1999) show it to be uniquely long, although decidedly
cooler than the maximum temperatures of the previous four interglacials,
at least in continental Antarctica. Current anthropogenic global warming
via greenhouse gasses threatens to elevate world temperatures to levels
that in past interglacials apparently triggered a large feedback effect
producing a relatively rapid decline toward glacial conditions. The Arctic
ocean ice pack is currently thinning very rapidly (Kerr 1999). A dark,
open Arctic Ocean would dramatically increase the heat income at high northern
latitudes, and have large, difficult to guess impacts on the Earth’s climate
system. No one can yet estimate the risks we are taking of a rapid return
to colder, drier, more variable environment with less CO2, nor
evaluate exactly the threat such conditions imply for the continuation
of agricultural production. Nevertheless, the intrinsic instability of
the Pleistocene climate system, and the degree to which agriculture is
dependent upon the unusually long Holocene stable period, should give one
pause (Broecker 1997).
REFERENCES CITED
Allen,
J. R., U. Brandt, A. Brauer, H.-W. Hubberten, B. Huntley, J. Keller, M.
Kraml, A. Mackensen, J. Mingram, J. F. Negendank, N. R. Nowaczyk, H. Oberhansli,
W. A. Watts, S. Wulf and B. Zolitschka
1999 Rapid Environmental Changes in Southern Europe During the Last Glacial
Period. Nature 400:740-743.
Ammerman,
A.J. and L.L. Cavalli-Sforza
1971 Measuring the Rate of Spread of Early Farming in Europe. Man
6:674-688.
An,
Z.
1991 Radiocarbon Dating and the Prehistoric Archaeology of China. World
Archaeology 23(2):193-100.
Barnola, J.M., D. Raynaud, Y.S. Korotkevich and C.
Loris
1987 Vostok Ice Core Provides 160,000-Year Record of Atmospheric CO2.
Nature 329: 408-414.
Bar-Yosef,
O, and R.H. Meadow
1995 The Origins of Agriculture in the Near East. In Last Hunters, First
Farmers: New Perspectives on the Prehistoric Transition to Agriculture,
edited by T.D. Price. and B. Gebauer, pp.39-94. School of American Research
Press, Santa Fe.
Bellwood,
P.
1996 The Origins and Spread of Agriculture in the Indo-Pacific Region:
Gradualism and Diffusion or Revolution and Colonization. In The Origins
and Spread of Agriculture and Pastoralism in Eurasia, edited by D.
R. Harris, pp. 465-498. Smithsonian Institution Press, Washington, D. C.
Bettinger,
R.L.
1991 Hunter-Gatherers: Archaeological and Evolutionary Theory. Plenum,
New York.
Bettinger,
R. L.
1999 From Traveler to Processor: Regional Trajectories of Hunter-Gatherer
Sedentism in the Inyo-Mono Region, California. In Settlement Pattern
Studies in the Americas: Fifty Years Since Viru, edited by B. R. Billman
and G. M. Feinman, pp. 39-55. Smithsonian Institution Press, Washington,
D.C.
Bettinger,
R.L.
2000 Holocene Hunter-gatherers. In Archaeology at the Millennium: A
Sourcebook, edited by G.M. Feinman and T.D. Price. Plenum, New York,
in press.
Bettinger,
R.L., and M.A. Baumhoff
1982 The Numic Spread: Great Basin Cultures in Competition. American
Antiquity 47: 485-503.
Bettinger,
R. L. and J. Eerkens
1999 Point Typologies, Cultural Transmission, and the Spread of Bow and
Arrow Technology in the Prehistoric Great Basin. American Antiquity
64:231-242.
Blumler,
M.
1992 Seed Weight and Environment in Mediterranean-type Grasslands in
California and Israel. Ph.D. Thesis, University of California, Berkeley.
UMI, Ann Arbor.
Boyd,
R. and P.J. Richerson
1985 Culture and the Evolutionary Process. University of Chicago
Press, Chicago.
Boyd,
R. and P.J. Richerson
1992 How Microevolutionary Processes Give Rise to History. In History
and Evolution, edited by M.H. and D.V. Nitecki, pp.179-209. State University
Press of New York, Albany.
Bradley,
R.S.
1999 Paleoclimatology: Reconstructing Climates of the Quaternary,
Second Edition. Academic Press, San Diego.
Braidwood,
R.J.
1960 The Agricultural Revolution. Scientific American 203(3):130-148.
Braidwood,
R,J. and B. Howe
1960 Prehistoric Investigations in Iraqi Kurdistan. Studies in Ancient
Oriental Civilization 31. University of Chicago Oriental Institute, Chicago.
Braidwood,
L., R. Braidwood, B. Howe, C. Reed, and P.J. Watson (editors)
1983 Prehistoric Archaeology Along the Zagros Flanks. Studies in
Ancient Oriental Civilization 105. University of Chicago Oriental Institute,
Chicago.
Bretting
P.K. (editor)
1990 New Perspectives on the Origin and Evolution of New World Domesticated
Plants. Economic Botany 44 (Supplement).
Broecker,
W.S.
1997 Thermohaline Circulation, the Achilles Heel of Our Climate System:
Will Man-Made CO2 Upset the Current Balance? Science
178:1582-1588.
Broecker,
W.S.
1996 Glacial Climate in the Tropics. Science 272: 1902-1903.
Brush,
S.
1995 In Situ Conservation of Landraces in Centers of Crop Diversity.
Crop Science 35:346-353.
Carroll,
R.L.
1997 Patterns and Processes in Vertebrate Evolution. Cambridge University
Press, Cambridge.
Cavalli-Sforza,
L.L., P. Menozzi, and A. Piazza
1994 The History and Geography of Human Genes. Princeton University
Press, Princeton.
Childe,
V.G.
1951 Man Makes Himself. Watts, London.
Clark,
P.U., R.B. Alley, and D. Pollard
1999 Northern Hemisphere Ice-Sheet Influences on Global Climate Change.
Science 286:1104-1111.
Cohen,
M.N.
1977 The Food Crisis in Prehistory: Overpopulation and the Origins of
Agriculture. Yale University Press, New Haven.
Cohen,
M.N. and G.J. Armelagos
1984 Paleopathology at the Origins of Agriculture. Academic Press,
Orlando.
Crawford,
G. W.
1992 Prehistoric Plant Domestication in East Asia. In The Origins
of Agriculture, edited by C. W. Cowan and P. J. Watson, pp. 7-38. Smithsonian
Institution Press, Washington, D.C.
Crosby,
A.W.
1986 Ecological Imperialism: The Biological Expansion of Europe, 900-1900.
Cambridge University Press, Cambridge.
Crowley,
T.J. and G.R. North
1991 Paleoclimatology. Oxford University Press, New York.
Darwin,
C.
1902 [1874] The Descent of Man and Selection and Selection in Relation
to Sex. American Home Library, New York.
Day,
R.H. and J-L. Walter
1989 Economic Growth in the Very Long Run: On the Multiple Phase Interaction
of Population, Technology, and Social Infrastructure. In Economic Complexity:
Chaos, Sunspots, Bubbles, and Nonlinearity, edited by W.A. Barnett,
J. Geweke, and K. Schell, pp. 253-289. Cambridge University Press, Cambridge.
Diamond,
J.
1997 Guns, Germs, and Steel. Norton, New York.
Ditlevsen,
P.D., H. Svensmark, and S. Johnsen
1996 Contrasting Atmospheric and Climate Dynamics of the Last-glacial and
Holocene Periods. Nature 379: 810-812.
Durham,
W.H.
1991 Coevolution: Genes, Culture, and Human Diversity. Stanford
University Press, Stanford.
Easterlin,
R.A.
1995 Will Raising the Incomes of All Increase the Happiness of All? Journal
of Economic Behavior and Organization 27:35-47.
Ehret,
C.
1998 An African Classical Age: Eastern and Southern Africa in World
History, 1,000 B.C. to A.D. 400. University of Virginia Press, Charlottesville.
Eldredge,
N. and S.J. Gould
1972 Punctuated Equilibria: An Alternative to Phyletic Gradualism. In Models
in Paleobiology, edited by T.J.M. Schopf, pp. 82-115. San Francisco:
Freeman.
Elston,
R. G., C. Xu, D. B. Madsen, K. Zhong, R. L. Bettinger, J. Li, P. J. Brantingham,
H. Wang and J. Yu
1997 New Dates For the North China Mesolithic. Antiquity 71:985-993.
Endler,
J.A.
1986 Natural Selection In the Wild. Princeton University Press,
Princeton.
Fairbank,
J.K.
1992
China: A New History. Harvard University Press, Cambridge MA.
Fiedel,
S.J.
1999 Older Than We Thought: Implications of Corrected Dates for Paleoindians.
American Antiquity 64:95-115.
Flannery,
K.V.
1973 The Origins of Agriculture. Annual Review of Anthropology 2:271-310.
Frank,
R.H. and P.J. Cook
1995 The Winner-Take-All Society. Free Press, New York.
Frogley,
M.R., Tzedakis, P.C., and T.H.E. Heaton
1999 Climate Variability in Northwestern Greece During the Last Interglacial.
Science 285: 886-1889.
Gifford-Gonzales,
D.
In press. Animal Disease Challenges to the Emergence of Pastoralism in
Sub-Saharan Africa. African Archaeological Review.
Goring-Morris,
N., and A. Belfer-Cohen
1998 The Articulation of Cultural Processes and Late Quaternary Environmental
Change In Cisjordan. Paléorient 23:71-93.
GRIP
(Greenland Ice-core Project Members)
1993 Climate Instability During the Last Interglacial Period Recorded In
the GRIP Ice Core. Nature 364:203-207.
Harris,
D.R. (editor)
1996 The Origins and Spread of Agriculture and Pastoralism In Eurasia.
University College London Press, London.
Harris,
D.R.
1977 Alternative Pathways Toward Agriculture. In Origins of Agriculture,
edited by C.A. Reed, pp. 179-243. Mouton, The Hague.
Hayden,
B.
1995 A New Overview of Domestication. In Last Hunters, First Farmers:
New Perspectives on the Prehistoric Transition to Agriculture, edited
by T.D. Price and B. Gebauer, pp.273-299. School of American Research Press,
Santa Fe.
Henry,
D.O.
1989 From Foraging to Agriculture: The Levant At the End of the Ice
Age. University of Pennsylvania Press, Philadelphia.
Imamura,
K.
1996 Jomon and Yoyoi: The Transition to Agriculture in Japanese Prehistory.
In The Origins and Spread of Agriculture and Pastoralism in Eurasia,
edited by D.R. Harris, pp. 442-464. University College London Press, London.
Johnson,
T.C., C.A. Scholz, M.R. Talbot, K. Ketts, R.D. Ricketts, G. Ngobi, K. Buening,
I. Ssemmanda and J.W. McGill
1996 Late Pleistocene Dessication of Lake Victoria and Rapid Evolution
of Cichlid Fishes. Science 273: 1091-93.
Lamb,
H.H.
1977 Climatic History and the Future. Princeton University Press,
Princeton.
Lee,
R.D.
1986 Malthus and Boserup: A Dynamic Synthesis. In The State of Population
Theory: Forward From Malthus, edited by D. Coleman and R. Schofield,
pp. 96-130. Basil Blackwell, Oxford.
Levinton,
J.
1988 Genetics, Paleontology, and Macroevolution. Cambridge University
Press, Cambridge.
Lindert,
P.H.
1985 English Population, Wages, and Prices: 1541-1913. Journal of Interdisciplinary
History 15: 609-34.
Katz,
S.H., M.L. Hediger, and L.A. Valleroy
1974 Traditional Maize Processing Techniques in the New World. Science
184: 765-773.
Kent
J.D.
1987 Periodic Aridity and Prehisanic Titicaca Basin Settlement Patterns.
In Arid Land Use Strategies and Risk Management in the Andes, edited
by D.L. Browman, pp 297-314. Westview, Boulder. .
Kerr,
R.A.
1999 Will the Arctic Ocean Lose All Its Ice? Nature 286: 128.
Klein,
R. G.
1999 The Human Career: Human Biological and Cultural Origins. University
of Chicago Press, Chicago.
Kirch,
P.V.
1984 The Evolution of Polynesian Chiefdoms. Cambridge University
Press, Cambridge.
Lindsay,
L. M.
1986 Fremont Fragmentation. In Anthropology of the Desert West: Essays
in Honor of Jesse D. Jennings, edited by C. J. Condie and D. D. Fowler,
pp. 229-252. Anthropological Papers No. 110. University of Utah, Salt Lake
City.
MacNeish,
R.S.
1973 The Evolution of Community Patterns in the Tehuacán Valley
of Mexico and Speculations About the Cultural Process. In Ecology and
Agricultural Settlements: An Ethnographic and Archaeological Perspective,
edited by R. Tringham, pp. 43-55. Andover MA: Warner Modular Publications,
Andover MA.
MacNeish,
R.S.
1991 The Origins of Agriculture and Settled Life. University of
Oklahoma Press: Norman.
Matson,
R. G.
1999 The Spread of Maize to the Colorado Plateau. Archaeology Southwest
13:10-11.
McNeill,
W.H.
1976 Plagues and Peoples. Anchor, Garden City NY.
Martin,
P.S., and R.G. Klein (editors)
1984 Quaternary Extinctions: A Prehistoric Revolution. University
of Arizona Press, Tucson.
Mayewski,
P. A., M. L. D., W. S, M. S. Twickler, M. C. Morrison, R. B. Alley, P.
Bloomfield and K. Taylor
1993 The Atmosphere During the Younger Dryas. Science 261:195-100.
Murray,
J. D.
1989 Mathematical Biology. Springer-Verlag, Berlin.
North,
D.C. and R.P. Thomas
1973 The Rise of the Western World: A New Economic History. Cambridge
University Press, Cambridge.
Partridge,
T.C., G.C. Bond, C.J.H. Hartnady, P.B. deMenocal, and W.F. Ruddiman
1995 Climatic Effects of Late Neogene Tectonism and Vulcanism. In Paleoclimate
and Evolution With Emphasis on Human Origins, edited by E. S. Vrba,
G. H. Denton, T. C. Partridge, L. H. Burckle, pp. 8-23. Yale University
Press, New Haven.
Petit,
J.R., J. Jouzel, D. Raynaud, N.I. Barkov, J.-M. Barnola, J. Basile, M.
Bender, J. Cappellaz, M. Davis, G. Delaygue, M. Delmotte, V.M. Kotlyakov,
M. Legrand, V.Y. Lipenkov, C. Lorius, L. Pépin, C. Ritz. E. Saltzman,
and M. Stievenard
1999 Climate and Atmospheric History of the Past 420,000 Years From the
Vostok Ice Core, Antarctica. Nature 399:429-436.
Price,
T.D. and B. Gebauer
1995 Last Hunters, First Farmers: New Perspectives on the Prehistoric
Transition to Agriculture. School of American Research Press, Santa
Fe.
Richerson,
P.J. and R. Boyd
1998 The Evolution of Human Ultrasociality. In Indoctrinability, Ideology,
and Warfare: Evolutionary Perspectives, edited by I. Eibl-Eibesfeldt
and F.K. Salter, pp. 71-95. Berghahn, New York.
Richerson,
P.J. and R. Boyd
1999 Complex Societies: The Evolutionary Origins of a Crude Superorganism.
Human Nature 10:253-289.
Richerson,
P.J. and R. Boyd.
2000 Climate, Culture, and the Evolution of Cognition. In Evolution
of Cognition, edited by C. Heyes and L. Huber. MIT Press, Cambridge
MA, in press.
Rindos,
D.
1984 The Origins of Agriculture: An Evolutionary Perspective. Academic
Press, London.
Rogers,
E.M.
1983 Diffusion of Innovations, 3rd Edition. Free Press,
New York.
Sage,
R.F.
1995 Was Low Atmospheric CO2 During the Pleistocene a Limiting
Factor For the Origin of Agriculture? Global Change Biology 1:93-106.
Simpson,
G.G.
1944 Tempo and Mode in Evolution. Columbia University Press, New
York.
Smith,
B.D.
1989 Origins of Agriculture in Eastern North America. Science 246:
1566-1571.
Smith,
B.D.
1995 The Emergence of Agriculture. Scientific American Library,
New York.
Smith,
B. D.
1997 Initial Domestication of Curcurbita pepo in the Americas 10,000
Years Ago. Science 276: 932-934.
Steward,
J.H.
1955 Theory of Culture Change: The Methodology of Multilinear Evolution.
University of Illinois Press, Urbana.
Steig,
E.J., E.J. Brook, J.W.C. White, C.M. Sucher, M.L. Bender, S.J. Lehman,
D.L. Morse, E.D. Waddington, and G.D. Glow
1998 Synchronous Climate Changes in Antarctica and the North Atlantic.
Science 282: 92-95.
Stuiver,
M., P. J. Reimer, E. Bard, J. W. Beck, G. S. Burr, K. A. Hughen, B. Kromer,
G. McCormack, J. V. D. Plicht and M. Spurk
1998 INTCAL98 Radiocarbon Age Calibration, 24,000 - 0 cal BP. Radiocarbon
40:1041-1083.
Tainter,
J. A.
1988 The Collapse of Complex Societies. Cambridge University Press,
Cambridge.
Valentine,
J.W. and E.M. Moores
1970 Plate-Tectonic Regulation of Faunal Diversity and Sea Level: A Model.
Nature 228: 657-659.
Vayda,
A.P.
1995 Failures of Explanation in Darwinian Ecological Anthropology: Part
II. Philosopy of the Social Sciences 25: 360-375.
Von
Grafenstein, U., H. Erlenkeuser, A. Brauer, J. Jouzel, and S.J. Johnsen
1999 A Mid-European Decadal Isotope-Climate Record From 15,500 to 5,000
years B.P. Science 284: 1654-1657.
Wohlgemuth,
E.
1996 Resource Intensification In Prehistoric Central California: Evidence
From Archaeobotanical Data. Journal of California and Great Basin Archaeology
18: 81-103.
Walker,
T.D. and J.W. Valentine
1984 Equilibrium Models of Evolutionary Species Diversity and the Number
of Empty Niches. American Naturalist 124: 887-899.
Wiessner,
P., and A. Tumu
1998 Historical Vines: Enga Networks of Exchange, Ritual, and Warfare
in Papua New Guinea. Smithsonian Institution Press, Washington D.C.
Winterhalder,
B.
1986 Diet Choice, Risk, and Food Sharing in a Stochastic Environment. Journal
of Anthropological Archaeology 5:369-392.
Woodburn,
J.
1980 Hunter-Gatherers Today and Reconstruction of the Past. In Soviet
and Western Anthropology, edited by E. Gellner, pp. 95-117. Columbia
University Press, New York.
Wright,
H.E., Jr.
1977 Environmental Change and the Origin of Agriculture In the Old and
New Worlds. In Origins of Agriculture, edited by C.A. Reed, pp.
281-318. Mouton, The Hague.
Zohary,
D., and M. Hopf
1993 Domestication of Plants in the Old World: The origin and Spread
of Cultivated Plants in West Asia, Europe, and the Nile Valley, 2nd
Edition. Oxford University Press, Oxford.
Zvelebil,
M.
1996 The Agricultural Frontier and the Transition to Farming in the Circum-Baltic
Region. In The Origins and Spread of Agriculture and Pastoralism in
Eurasia, edited by D. R. Harris, pp.323-345. Smithsonian Institution
Press, Washington, D.C.